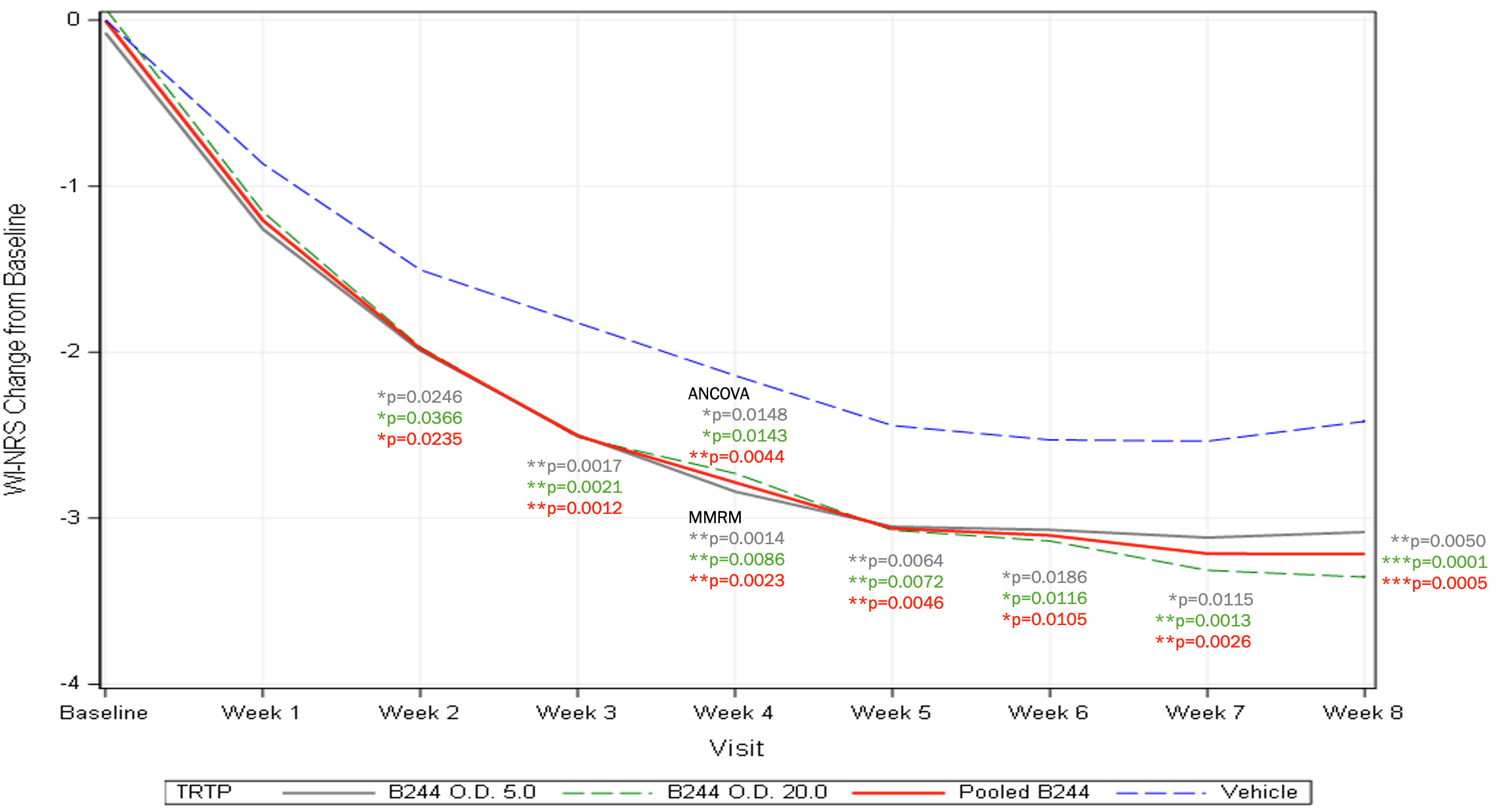
- B244 reduced patients’ WI-NRS score by an average of 34.3% and achieved clinically meaningful 4-point itch response
- Lesional severity (appearance) improved for IGA and EASI
- Results were achieved in 4 weeks
- Safe and well tolerated with no SAEs and no single adverse event occurred in greater than 1% of the active group.
CAMBRIDGE, Mass., February 22, 2022 /PRNewswire/ – AOBiome Therapeutics, a clinical stage biotechnology company focused on transforming human health by developing topical biologic therapies for systemic inflammatory conditions, announced positive pivotal Phase 2b results from its trial evaluating B244 in both pruritus (itch) and appearance of atopic dermatitis (eczema). The trial enrolled 547 patients with mild-to-moderate appearance of atopic dermatitis and moderate-to-severe itch. The trial met all primary and secondary endpoints, showing that B244 significantly reduced itch, reduced overall disease severity, improved skin clearance, and improved health-related quality of life measures at 4 weeks compared to placebo.
- B244 reduced enrolled patients’ mean Worst Itch Numeric Rating Scale (WI-NRS) score by 34.3% (-2.8 B244 vs -2.1 placebo, p=0.0143 and p=0.0148 for high and low dose) from a baseline WI-NRS score above 8 (out of 10).
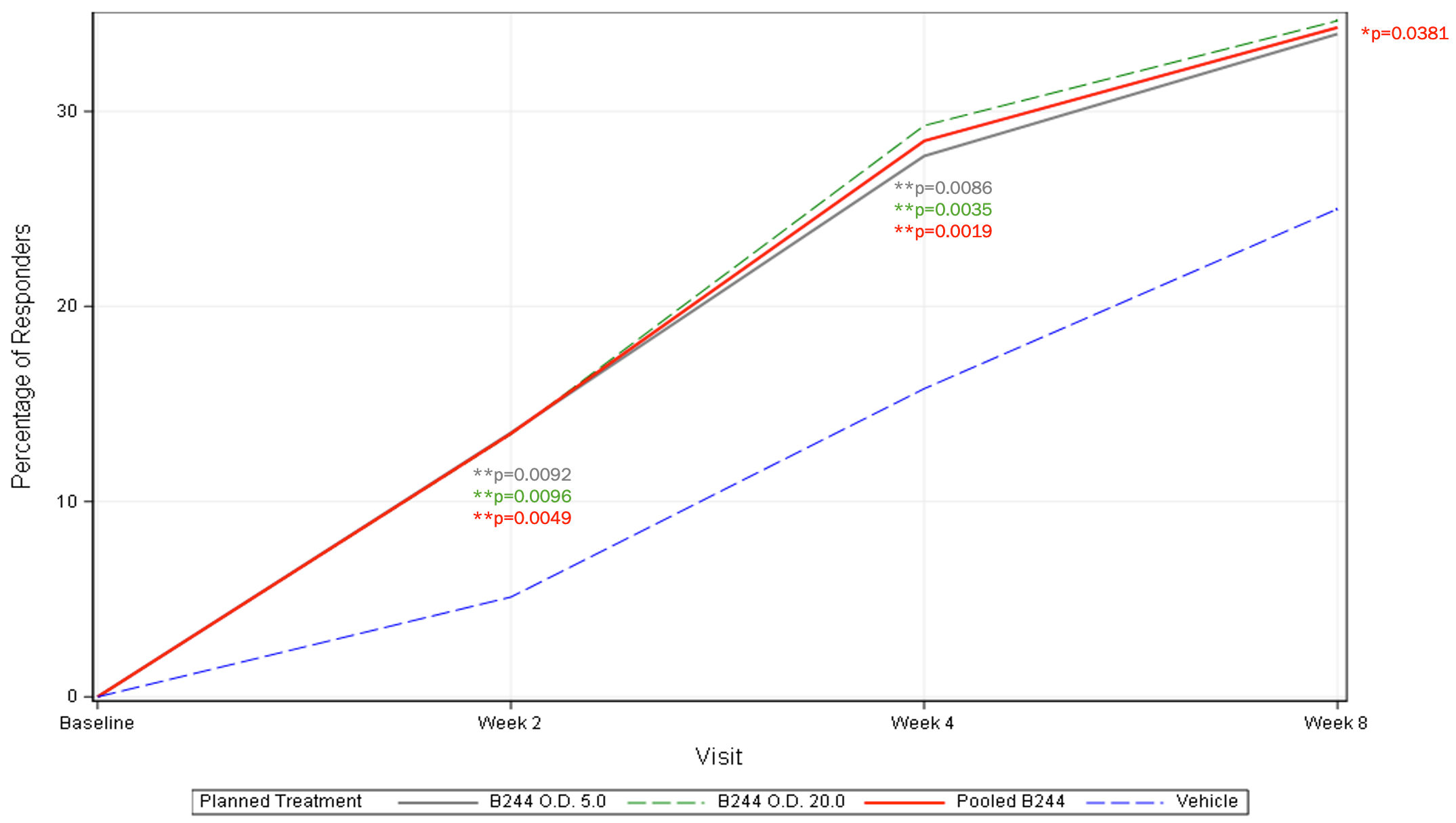
- Secondary endpoints related to appearance also achieved statistical significance. 29.3% and 27.7% of patients in the high and low dose groups of B244 achieved EASI-75 success vs. 15.8% in the placebo group (p=0.0035 and p=0.0086, respectively) and 26.2% and 21.7% of patients in the high and low dose groups of B244 achieved IGA success (≥2-point improvement in IGA to clear or almost clear) vs. 12.3% in the placebo group (p=0.0015 and p=0.0228, respectively).
- B244 was well tolerated with no SAEs; treatment related treatment emergent adverse events (TEAEs) were low in incidence, mild in severity, and transient. The most common treatment related event was application site pruritus (0.8%).
“The favorable profile demonstrated in this study for safety and efficacy on both skin lesions and itch fills important unmet needs for first line therapies in atopic dermatitis. Current therapies often do not work or come with significant side effect risks. The efficacy observed for this topical spray after only 4 weeks is exciting for both clinicians and patients. An effective non-steroidal, non-injectable therapy without black box warnings or laboratory monitoring is pretty much the holy grail of unmet need in dermatology” said Dr. Jonathan Silverberg, Associate Professor of Dermatology, George Washington School of Medicine and Health Services.
Mean Change in WI-NRS (mITT) – 4 Week Treatment
EASI 75% Improvement (mITT) – 4 Week Treatment
“Topical biotherapeutic drug development is challenging, as traditional factors like PK/PD and animal models are not informative. We were able to isolate the immunomodulation of B244 across Il-4, 5, 13 and 31 and then select a dose and design a delivery regimen that highlighted the benefits of B244, while minimizing the high placebo rate that often plagues these types of studies. We are excited about moving toward a Phase 3 trial” said Todd Krueger, AOBiome’s President and CEO.
About the Phase 2b Clinical Trial
This trial was a double blind, randomized, placebo controlled, multicenter, Phase 2b dose selection study to evaluate the efficacy, safety, and tolerability of B244 live biotherapeutic topical spray twice daily for 28 days for the treatment of pruritus associated with atopic dermatitis in 547 adults with a history of mild-to-moderate atopic dermatitis (eczema) and moderate to severe pruritus (itch). The study was conducted at 56 U.S. sites across 25 states. The primary endpoint for the study was mean change in WI-NRS from baseline to week four. Secondary endpoints includedproportion of subjects with ≥4 point improvement in WI-NRS from baseline to week four. The itch WI-NRS scale is a validated, self-reported instrument for measurement of itch intensity. Additionally, endpoints of Investigator Global Assessment (IGA) and Eczema Area and Severity Index (EASI) for Atopic Dermatitis were captured.
Additional information regarding this and AOBiome’s other clinical programs may be found at www.clinicaltrials.gov.
About Atopic Dermatitis
In the United States, 7% of adults and 12% of children under the age of 18 years suffer from eczema and associated pruritus.1 Of these, approximately two thirds have mild to moderate cases. Patients with mild to moderate atopic dermatitis can exhibit moderate to severe pruritus which is inadequately addressed by available therapies. There is a significant unmet need to adequately treat this patient population with a safe and effective alternative to existing therapies.
About B244
AOBiome’s B244 platform is a patented, proprietary, topical and intranasal formulation. Once deployed, B244 produces nitric oxide, a signaling molecule known to regulate inflammation and vasodilation. B244 has been observed to be well-tolerated in clinical studies to date.
Additionally, recently published immunology data demonstrates that B244 can reduce the inflammatory and pruritic cytokines IL-4, IL-5, IL-13, and IL-31. See full article at: https://www.nature.com/articles/s41598-021-93299-1.
About AOBiome Therapeutics, Inc.
AOBiome Therapeutics, Inc. is a Cambridge, MA-based life sciences company focused on transforming human health by developing topical biologic therapies for dermatological, ocular, nasal, and systemic inflammatory conditions. Learn more at www.aobiome.com.
Contacts:
For Media Inquiries:
Jim Hoffman
845-417-3487
Jim@AOBiome.com
1 Hanifin J, Reed M. A Population-Based Survey of Eczema Prevalence in the United States. Dermatitis. 2007;18(2):82-91. doi:10.2310/6620.2007.06034.
| SOURCE AOBiome Therapeutics, Inc. |