- The newly issued patent covers Composition of Matter for AOBiome’s proprietary strain of Ammonia Oxidizing Bacteria (AOB), along with additional claims pertaining to a variety of related methods including use thereof in changing a composition of a skin microbiome of a subject, inhibiting microbial growth on a subject’s skin, and treating or preventing a skin disorder.
- AOBiome’s clinical candidate B244 has demonstrated clinical efficacy on multiple therapeutic indications including: Mild-to-Moderate Atopic Dermatitis (Eczema) and associated Moderate-to-Severe Pruritus (Itch), and mild-to-moderate Acne Vulgaris (Acne)
- AOBiome is currently preparing for its Global Phase 3 trial for the treatment of Atopic Dermatitis and associated Pruritus, as well as a Phase 2 study in moderate to severe Acne Vulgaris
CAMBRIDGE, Mass., October 2, 2024 /EIN Presswire/ — AOBiome Therapeutics, Inc. (“AOBiome”), a leading clinical-stage biotech company focusing on inflammatory conditions, today announced that the United States Patent Office (USPTO) has issued a composition of matter patent, U.S. Patent No. 12,091,652, with claims focused on its proprietary strain of beneficial AOB (Ammonia Oxidizing Bacteria), in the class of Nitrosomonas eutropha. The base patent term extends until November 3, 2035, excluding patent term extensions or coverage in additional related patent filings.
AOBiome’s B244 proprietary strain is delivered via a simple to use topical spray. Once deployed on the skin, AOB convert ammonia to nitrite, which is known to have antibacterial properties, and to nitric oxide, a signaling molecule known to regulate inflammation and vasodilation. It has also been demonstrated that this strain of AOB can reduce the inflammatory and pruritic cytokines, including IL-4, IL-5, IL-13, and IL-31 which are hallmarks of atopic response.
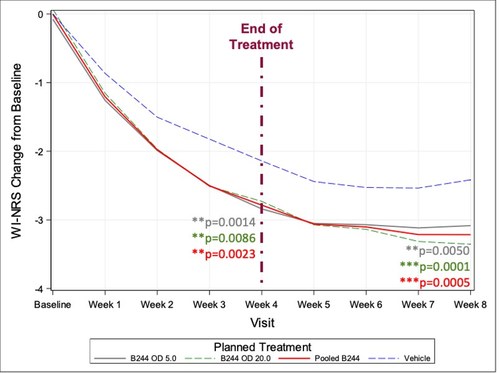
The Company completed a Phase 2b study of 547 patients with mild-to-moderate atopic dermatitis and associated moderate-to-severe pruritus. B244 showed continued durability of treatment effect and separation from placebo post-treatment of 41.1% reduction in itch from baseline at 8 weeks, 4 weeks after the last dose. This and other clinical trials have also demonstrated the unique safety profile of B244, with no treatment related SAE’s reported on any of the over 1,000+ patients that have participated in its trials.
This issuance follows the granting of the patent in numerous non-US territories including: Australia, Canada, China, France, Germany, Hong Kong, India, Japan, Korea and United Kingdom. The composition of matter patent gives an extra measure of protection to the already issued use patents that cover the clinical areas the company is pursuing, including Acne, Atopic Dermatitis, Rosacea, Psoriasis and other inflammatory skin diseases. AOBiome has also been granted other patents, in the US and internationally, covering manufacturing, quality assurance and product delivery.
“Getting a composition of matter patent speaks to the unique aspects of this proprietary strain of AOB which has shown superior growth, stability and metabolic activity when compared to other strains. This coupled with our existing issued use patents for the entire class of AOB gives us a unique position in the marketplace” says President & CEO, Todd Krueger.
AOBiome is currently preparing for its Global Phase 3 trial for the treatment of Atopic Dermatitis (Eczema) and associated Pruritus (Itch). The Phase 3 trial will include 2 arms of 934 each with a 900 person OLE. The trial will study both Adults and Children down to the age of 12 years. A follow-on study is planned for children down to the of 3 months. The trial will be run in the US, Europe and Japan in conjunction with AOBiome’s licensing partner Maruho.
About B244
AOBiome’s B244 platform is a patented, proprietary, topical formulation. Once deployed, B244 produces nitric oxide, a signaling molecule known to regulate inflammation and vasodilation. B244 has been observed to be safe and well-tolerated in clinical studies to date.
Additionally, recently published immunology data demonstrates that B244 can reduce the inflammatory and pruritic cytokines IL-4, IL-5, IL-13, and IL-31. See full article at: https://www.nature.com/articles/s41598-021-93299-1.
About AOBiome Therapeutics, Inc.
AOBiome Therapeutics, Inc. is a Cambridge, MA-based life sciences company focused on transforming human health by developing topical therapeutics for inflammatory conditions. AOBiome is advancing a pipeline of multiple, clinical-stage therapeutic candidates. Learn more at www.aobiome.com.
Contacts:
For Media Inquiries:
Jim Hoffman
+1.617.639.9980
Press@AOBiome.com